Ruptured abdominal aortic aneurysm (AAA) is one of the most fatal surgical emergencies, with an overall mortality rate of 90%. Most AAAs rupture into the retroperitoneal cavity, which results in the classical triad of pain, hypotension, and a pulsatile mass. However, this triad is seen in only 25–50% of patients, and many patients with ruptured AAA are misdiagnosed. It is likely that different sites of rupture of AAA determine a variety of common and uncommon clinical presentations, the recognition of which can save many lives.
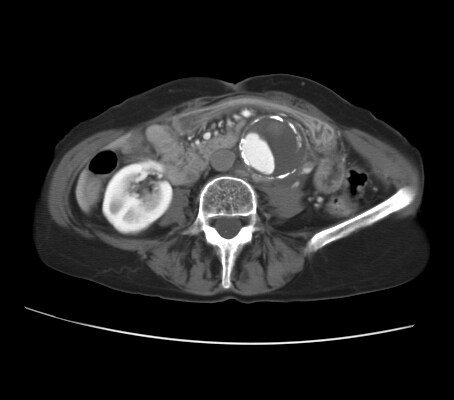
CT reveals an abdominal aortic aneurysm (AAA) with eccentric mural thrombus.
A disruption of the calcific rim of the AAA toward the left quadrant appears with adjacent isoattenuating soft tissue anterior to the left psoas muscle.
Clinical and radiologic findings are consistent with a diagnosis of contained AAA rupture with left retroperitoneal hematoma
http://emedicine.medscape.com/article/416397-overview


http://www.oup.com/uk/orc/bin/9780198569466/01student/cases/images/pic002.jpg
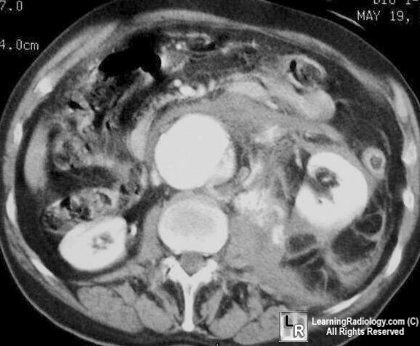

http://www.learningradiology.com/notes/cardiacnotes/aaapage.htm




http://radiographics.rsna.org/content/20/3/725/F44.large.jpg

CT reveals an abdominal aortic aneurysm (AAA) with eccentric mural thrombus.
A disruption of the calcific rim of the AAA toward the left quadrant appears with adjacent isoattenuating soft tissue anterior to the left psoas muscle.
Clinical and radiologic findings are consistent with a diagnosis of contained AAA rupture with left retroperitoneal hematoma
http://emedicine.medscape.com/article/416397-overview
Limitation of techniques
CT or MRI can rapidly provide detailed information about the blood vessels and their surrounding structures for treatment planning; the choice between them can be based on which is faster locally. Occasionally, however, these examinations may require too much time for them to be suitable for use in patients whose condition is unstable. Also, when contrast material is used in conjunction with CT to delineate blood-filled structures, it poses a risk of acute renal failure, particularly in hypovolemic elderly patients who may already have baseline nephrosclerosis or diabetic nephropathy.
Sonography is a quick and convenient modality, but it is much less sensitive and specific for the diagnosis of aneurysmal rupture. The absence of sonographic evidence of rupture does not rule out this entity if clinical suspicion is high.

non-contrast CT shows a large AAA and extensive periaortic haematoma. A thick (but subtle) hyperdense crescent is present within the aortic wall posteriorly and laterally which represents acute intramural haematoma, a sign of acute or impending rupture.
A ruptured AAA carries a 60 to 90% mortality before the patient reaches hospital and a 30 to 80% operative mortality for those who make it to surgery. The risk of rupture is proportional to the size and rate of growth of the aneurysm. Aneurysms greater than 5cm diameter or that grow faster than 10mm per year have a significantly increased risk of rupture and are indications for elective operative repair.
References:
1. Dahnert W. Radiology Review Manual, 5th edition. Lippincott, Williams and Wilkins 2003
2. Rakita, D. et al Spectrum of CT Findings in Rupture and Impending Rupture of Abdominal Aortic Aneurysms,Radiographics 2007;27:497-507
Credit: Dr Donna D'Souza http://www.radpod.org/2007/12/11/ruptured-abdominal-aortic-aneurysm/1. Dahnert W. Radiology Review Manual, 5th edition. Lippincott, Williams and Wilkins 2003
2. Rakita, D. et al Spectrum of CT Findings in Rupture and Impending Rupture of Abdominal Aortic Aneurysms,Radiographics 2007;27:497-507

http://www.oup.com/uk/orc/bin/9780198569466/01student/cases/images/pic002.jpg

Abdominal Aortic Aneurysm
- Focal widening >3 cm
- Normal size of abdominal aorta >50 years of age:
- About 2 cm
- Prevalence:
- Increases with age
- Greater with atherosclerotic disease
- Male predominance
- Whites: Blacks = 3:1
- Risk factors:
- male
- age >75 years
- white race
- prior vascular disease
- hypertension
- cigarette smoking
- family history
- hypercholesterolemia
- Associated with:
- visceral + renal artery aneurysm (2%)
- isolated iliac + femoral artery aneurysm (16%)
- common iliac (89%), internal iliac (10%), external iliac (1%)
- stenosis / occlusion of celiac trunk / SMA (22%)
- stenosis of renal artery (22-30%)
- occlusion of inferior mesenteric artery (80%)
- occlusion of lumbar arteries (78%)
- Growth rate of aneurysm of 3-6 cm in diameter:
- 0.39 cm / year
- Clinical
- asymptomatic (30%)
- abdominal mass (26%)
- abdominal pain (37%)
- Location
- infrarenal (91-95%) with extension into iliac arteries (66-70%)
- Imaging findings
- Plain film
- mural calcification (75-86%)
- US:>98% accuracy in size measurement
- CT-non-contrast enhanced
- perianeurysmal fibrosis (10%), may cause ureteral obstruction
- "crescent sign" = peripheral high-attenuating crescent in aneurysm wall (= acute intramural hematoma) = sign of impending rupture
- CT-contrast-enhanced
- ruptured aneurysm
- anterior displacement of kidney
- extravasation of contrast material
- fluid collection / hematoma within posterior pararenal + perirenal spaces (see below)
- ruptured aneurysm
- Plain film

- free intraperitoneal fluid
- contained leak
- laminated mural calcification
- periaortic mass of mixed / soft-tissue density
- lateral "draping" of aneurysm around vertebral body
- Angio
- focally widened aortic lumen >3 cm
- apparent normal size of lumen secondary to mural thrombus (11%)
- mural clot (80%)
- slow antegrade flow of contrast medium
- Contained rupture = extraluminal hematoma / cavity
- absent parenchymal stain = avascular halo
- displacement + stretching of aortic branches
- Complications:
- Rupture (25%)
- into retroperitoneum: commonly on left
- into GI tract: massive GI hemorrhage
- into IVC: rapid cardiac decompensation
- Incidence: aneurysm <4 cm in 10%, 4-5 cm in 23%, 5-7 cm in 25%, 7-10 cm in 46%, >10 cm in 60%
- Symptoms of rupture
- sudden severe abdominal pain ± radiating into back
- faintness, syncope, hypotension
- Prognosis:64-94% die before reaching hospital
- Increased risk: size >6 cm, growth >5 mm / 6 months, pain + tenderness
- Peripheral embolization
- Infection
- Spontaneous occlusion of aorta
- Rupture (25%)
- Prognosis:17% 5-year survival without surgery
- 50-60% 5-year survival with surgery
- Treatment
- surgery recommended if >5 cm in diameter;
- 4-5% surgical mortality for nonruptured
- 30-80% for ruptured aneurysm
- Postoperative Complications
- Left colonic ischemia (1.6%) with 10% mortality
- Renal failure (14%)
- 0-8% mortality rate for elective surgery

http://www.learningradiology.com/notes/cardiacnotes/aaapage.htm





http://radiographics.rsna.org/content/20/3/725/F44.large.jpg
